Acronym
INTERACT-II
Name of the study
Intraperitoneal irinotecan with concomitant FOLFOX and bevacizumab for patients with unresectable colorectal peritoneal metastases – a phase II study.
Researchers and contact information
Principal Investigator:
Dr. J.W.A. (Pim) Burger, Catharina Ziekenhuis, Eindhoven
Catharina Hospital, Eindhoven:
Principal investigator:
Dr. J.W.A. (Pim) Burger
Coordinating Investigator:
Drs. V.C.J. (Vincent) van de Vlasakker
Research Heelkunde, Postbus 1350, 5602 ZA, Eindhoven
Email: vincent.vd.vlasakker @catharinaziekenhuis.nl
Phone: +31 402396351
Erasmus MC Cancer Institute, Rotterdam
Principal investigators:
Prof. dr. A.H.J. Mathijssen
Prof. dr. C. (Kees) Verhoef
Coordinating investigators:
N.A.D Guchelaar
Interne Oncologie, Translationele Farmacologie
Be-451, postbus 3000, 3015 GD, Rotterdam
Email: n.guchelaar@erasmusmc.nl
Phone: +31 10 70 39640
Summary study
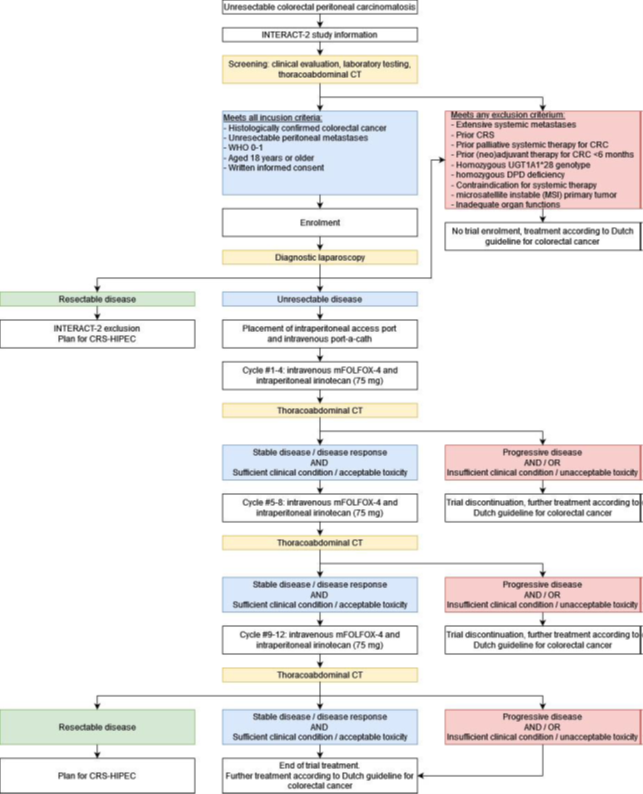
Rationale: The rationale of the current study is that the addition of intraperitoneal irinotecan (75 mg) to palliative systemic therapy is feasible and safe, and might result in an increased overall and progression free survival in patients with unresectable colorectal peritoneal metastases.
Objective: The primary objectives are to explore the overall survival for the addition of intraperitoneal irinotecan (75 mg) to palliative systemic therapy in patients with unresectable colorectal peritoneal metastases.
Secondary objectives are to assess the progression-free survival, toxicity profile, patient reported outcomes, costs, tumor response during trial treatment, and the systemic and intraperitoneal pharmacokinetics of irinotecan and SN-38.
Study design: This is a single-arm, open-label, phase II study that is performed in two Dutch tertiary referral centers for the surgical treatment of colorectal peritoneal metastases.
Study population: Patients are eligible for enrolment if they are adults who have unresectable colorectal peritoneal metastases, a WHO performance score of 0-1, and adequate organ functions. Patients are ineligible if they have extensive systemic metastases, have been treated with cytoreductive surgery or palliative systemic therapy for colorectal cancer, have a homozygous UGT1A1*28 genotype, or have any contra-indication for the planned chemotherapy.
Intervention: The addition of intraperitoneal irinotecan (75 mg) to modified FOLFOX4 (mFOLFOX4) + bevacizumab.
Main study parameters/endpoints: To determine the anti-tumor activity in patients treated with intraperitoneal irinotecan (75 mg) and concomitant mFOLFOX4, defined as (1) progression-free survival (calculated from the interval from the start of trial treatment until first evidence of intraperitoneal and/or systemic disease progression or last follow-up); (2) overall survival (calculated from (a) the interval from diagnosis of peritoneal metastases until death or last follow-up; (b) the interval from the first day of the first cycle until death or last follow-up). Nature and extent of the burden and risks associated with participation, benefit and group relatedness: Trial participation involves several potential (small) risks. Firstly, during the diagnostic laparoscopy a peritoneal port is placed, which is associated with a small risk of surgical complications. Secondly given the addition of a cytostatic agent, the most important risk associated with trial participation involves an increased risk of locoregional systemic toxicity.
Intervention