Acronym
CAIRO 6
Name of the study
Perioperative systemic therapy and cytoreductive surgery with HIPEC versus upfront cytoreductive surgery with HIPEC alone for resectable colorectal peritoneal metastases: a multicentre, open-label, parallel-group, phase II-III, randomised superiority study.
Researchers and contact information
Researchers and contact information
Lead investigator:
Dr. I.H.J.T. (Ignace) de Hingh
E: ignace.d.hingh@catharinaziekenhuis.nl
Other principal investigators
Dr. P.J. (Pieter) Tanis
E: p.j.tanis@amc.uva.nl
Prof. dr. C.J.A. (Kees) Punt
E: c.punt@amc.uva.nl
Trial statistician
Dr. M.G.W. (Marcel) Dijkgraaf
E: m.g.dijkgraaf@amc.uva.nl
Coordinating investigator
Drs. C. (Checca) Bakkers
Research Heelkunde, Postbus 1350, 5602 ZA, Eindhoven
E: checca.bakkers@catharinaziekenhuis.nl
CAIRO6 phone: +31658729079
Summary study
Rationale
There is no consensus on the benefit of perioperative systemic therapy for patients who qualify for cytoreductive surgery with HIPEC for isolated resectable colorectal peritoneal metastases.
Primary objective
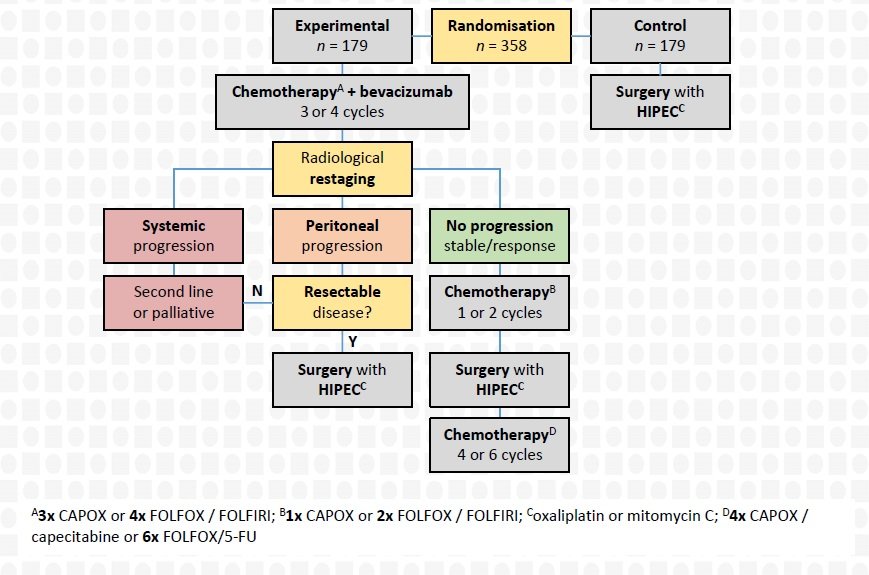
To compare the oncological efficacy of perioperative systemic therapy and cytoreductive surgery with HIPEC (experimental arm) versus upfront cytoreductive surgery with HIPEC alone (control arm) for isolated resectable colorectal peritoneal metastases.
Study design
Multicentre, open-label, parallel-group, phase II-III randomised superiority study with 1:1 computer-generated randomisation by stratified minimisation with peritoneal cancer index (≤10 versus >10), metastatic entity (synchronous versus metachronous), previous systemic therapy (yes versus no), and planned HIPEC agent (oxaliplatin versus mitomycin C) as stratification factors.
Study population
Adults with a good performance status (WHO), histological/cytological confirmation of a non-appendiceal colorectal carcinoma with <50% signet ring cells in peritoneal deposits or ascites, resectable disease confirmed by laparotomy or laparoscopy, no systemic metastases, no systemic therapy 6 months in the past 6 months, no resection of systemic metastases in the past 3 months, no contraindications for the planned systemic therapy regimens, and no previous cytoreductive surgery with HIPEC.
Primary endpoint
Overall survival 3 years after randomisation
Secondary endpoints
Major 90-day postoperative morbidity, progression-free survival, disease-free survival, quality of life, costs, systemic therapy related toxicity, objective radiological response rate, objective histological response rate
Sample size
The expected 3-year overall survival is 65% in the experimental arm and 50% in the control arm. A total of 358 patients (179 in each arm) are needed to detect such a difference with α 0.05, β 0.8, and a drop-out of 5%.
Intervention
Control arm
Upfront cytoreductive surgery with HIPEC
Experimental arm
Perioperative systemic therapy and cytoreductive surgery with HIPEC
Participating centers
- Antoni van Leeuwenhoek Ziekenhuis, Amsterdam
- Catharina Ziekenhuis, Eindhoven
- Erasmus Medisch Centrum, Rotterdam
- Medisch Spectrum Twente, Enschede
- Radboud Universitair Medisch Centrum, Nijmegen
- St. Antonius Ziekenhuis, Nieuwegein
- Universitair Medisch Centrum Groningen, Groningen
- Universitair Medisch Centrum Utrecht, Utrecht
- VU Medisch Centrum, Amsterdam